
The Lancet Commission on prostate cancer projects a rise in global prostate cancer cases from 1.4 million annually in 2020 to 2.9 million in 2040, with annual deaths increasing by 85% during this period, from 375,000 in 2020 to nearly 700,000 in 2040. Underdiagnosis in low - and middle - income countries and data collection gaps suggest the actual figures could be higher. Prostate cancer, a common male urogenital malignancy, significantly affects male health and quality of life. Its unique tumor microenvironment makes it an "immunological desert", limiting the effectiveness of immunotherapy. While traditional treatments like surgery, radiotherapy, and androgen deprivation therapy are effective initially, they are less so for advanced and metastatic prostate cancer.
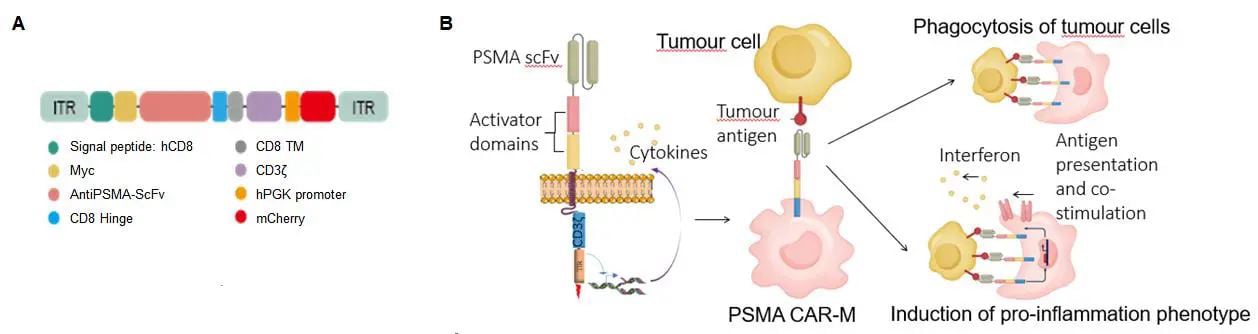
A recent paper titled "PSMA-specific CAR-engineered macrophages for therapy of prostate cancer", published on bioRixv by RocRock Bio's Yin Xiushan team and others, details the development of PSMA - specific CAR-M cells. These cells, engineered with a PSMA-specific single-chain variable fragment and co-stimulatory domain, demonstrated strong antitumor activity and safety in vitro and in vivo, providing a solid preclinical basis for future clinical tri

CAR-M cells are designed to recognize and eliminate tumor cells through genetic engineering of macrophages. In this study, researchers developed CAR-M cells targeting PSMA. These cells were engineered with a CAR construct that includes a PSMA-specific single-chain variable fragment (scFv) and costimulatory domains, enabling them to specifically target prostate cancer cells. In vitro experiments demonstrated that these CAR-M cells can effectively identify and destroy prostate cancer cells expressing PSMA, while exhibiting a strong pro-inflammatory response. This innovative approach opens new avenues for the immunotherapy of prostate cancer.
。

Research demonstrates the safety and efficacy of PSMA - targeted CAR-M cells in vivo. Histopathological and biochemical analyses reveal no significant damage to normal tissues, confirming their clinical safety. The consistent antitumor effects observed in both in vitro and in vivo studies highlight the promising prospects of CAR-M cells in prostate cancer treatment. This study represents the first global exploration of a PSMA - targeted CAR-M therapy for prostate cancer, paving the way for applications in advanced pancreatic cancer treatment. PSMA - targeted CAR-M cells have shown remarkable antitumor effects and favorable safety in prostate cancer models. Looking ahead, RocRock Bio will assess the safety and efficacy of prostate cancer CAR-M cells in clinical settings. RocRock Bio, a clinical - stage cell therapy company specializing in macrophage - based cancer treatments, co - launched an Investigator - Initiated Trial (IIT) of the RR-M01 pipeline with Xuzhou Medical College Affiliated Hospital in 2023. Multiple subjects have been dosed, proving RR-M01's safety and efficacy, and offering new hope for treating malignant solid tumors, particularly recurrent and refractory ovarian cancer. The RR-M01 pipeline is set to complete the last patient enrollment and dosing in October 2024. Its indications will expand to all solid tumors with HER2 positivity or low expression, approved by the hospital's ethics committee in early March 2024, with four subjects dosed and plans to recruit 20 patients in 2024. Clinical trials targeting pancreatic cancer and small cell lung cancer are also progressing smoothly.
No Code Website Builder