CAR-M cells, with their superior immune infiltration and efficient target-killing abilities, have shown significant clinical potential in solid tumor treatment. To promote the clinical translation and application of CAR-M therapy, developing an efficient and precise evaluation system to quickly measure its infiltration and killing performance is crucial. This will help understand the CAR-M mechanism and optimize treatment plans for better patient outcomes.
Organoid technology, by reducing animal experiments and using human tissue, avoids species differences and is ideal for patient-specific tumor research and infectious disease mechanism exploration. Additionally, organoids can form multi-organ systems and long-term cultures, providing a unique advantage for studying drug interactions across organs and long-term toxicity effects.
Recently, RocRock Bio, Pu-Heng-Bo-Mai, and Professor Zhang Ge's team collaborated to develop a high-throughput in vitro screening system for RocRock Bio's HER2-targeted CAR-M cells using Pu-Heng-Bo-Mai's 3D NAC-Organoid technology platform. Their research, titled "3D DNA Origami Assembled Spheroid for Evaluating Cytotoxicity and Infiltration of Chimeric Antigen Receptor Macrophage (CAR-M)," was published in Communications Biology. RocRock Bio is the first corresponding unit, with Professor Yin Xiushan, Dr. Song Guangqi, Professor Zhao Yicheng, and Professor Zhang Ge as co-corresponding authors.

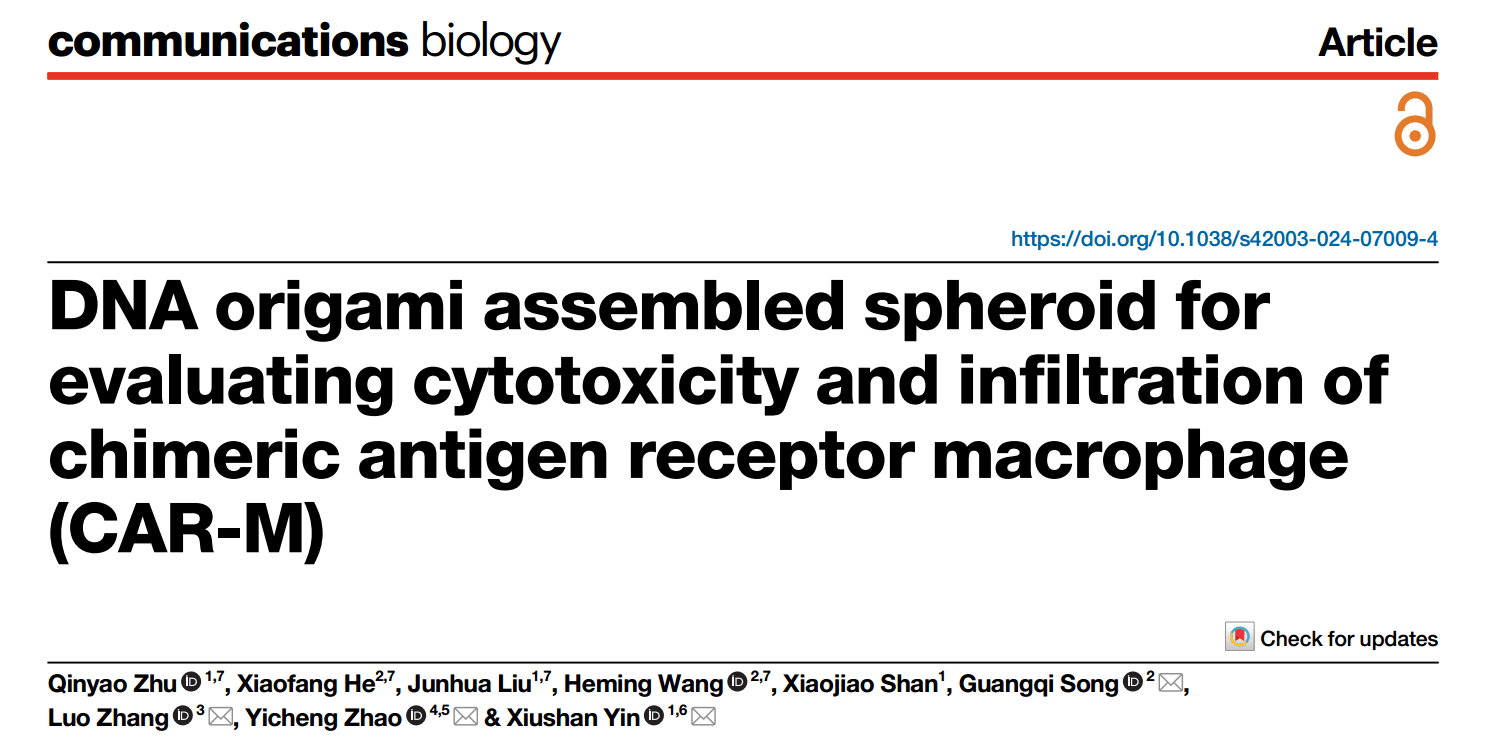
The research team designed and constructed a CAR gene cassette targeting HER2. By utilizing a virus vector with high gene transfer efficiency, they introduced the CAR-HER2 gene into human monocyte-derived macrophages (hMDM) and the mouse macrophage cell line RAW264.7, achieving a CAR-positive expression rate of over 85%. The study revealed that both hMDM CAR cells and RAW CAR cells, though having certain phenotypic differences, display a pro-inflammatory phenotype. This characteristic is beneficial for boosting the activity of HER2 CAR-M cells within the tumor microenvironment, thereby enhancing their ability to kill tumor cells.
To evaluate how effectively HER2 CAR-M cells can engulf and kill HER2-positive cells, we chose SKOV3 ovarian cancer cells, which have high HER2 expression, and SKOV3-fluc cells labeled with firefly luciferase as targets. Using a dual-color labeling method, we stained CAR-M cells and target cells separately. After co-culturing them, we assessed the phagocytic efficiency of CAR-M cells. Flow cytometry results revealed that both hMDM CAR cells and RAW CAR cells in the CAR-M group showed a much higher phagocytic efficiency for SKOV3 cells compared to the control group.
Moreover, we evaluated the cytotoxicity of HER2 CAR-M cells using two methods: an LDH release assay, which measures cytotoxicity by detecting LDH enzyme activity released from damaged cells, and firefly luciferase activity detection, which reflects cell survival changes by monitoring the decrease in luciferase activity. Both methods consistently demonstrated that HER2 CAR-M cells have a significantly stronger cytotoxic effect on SKOV3-Fluc cells.

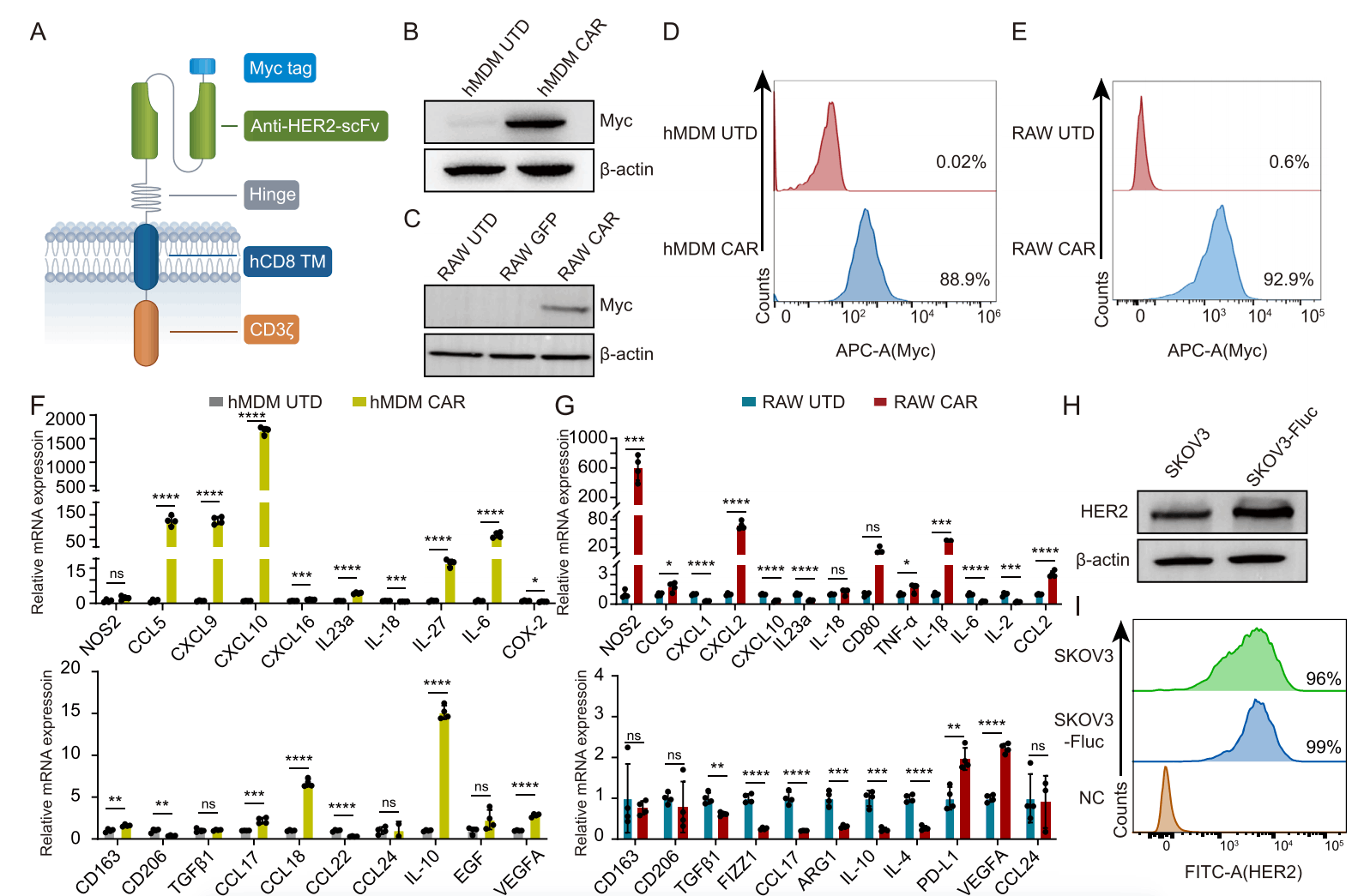
The research team applied their self-developed NAC-Organ technology to construct a 3D ovarian cancer spheroid (NAC-Solid Tumor) using SKOV3 cells. The model is highly stable and active, requires no matrix gel for support, and enables long-term co-culture with immune cells, allowing their active invasion into the tumor. Live-cell real-time imaging showed macrophages could effectively infiltrate this tumor model. This 3D tumor model successfully replicates the dynamic interaction between tumors and immune cells, offering a robust platform to assess CAR-M cells' targeting, infiltration, and cytotoxicity.
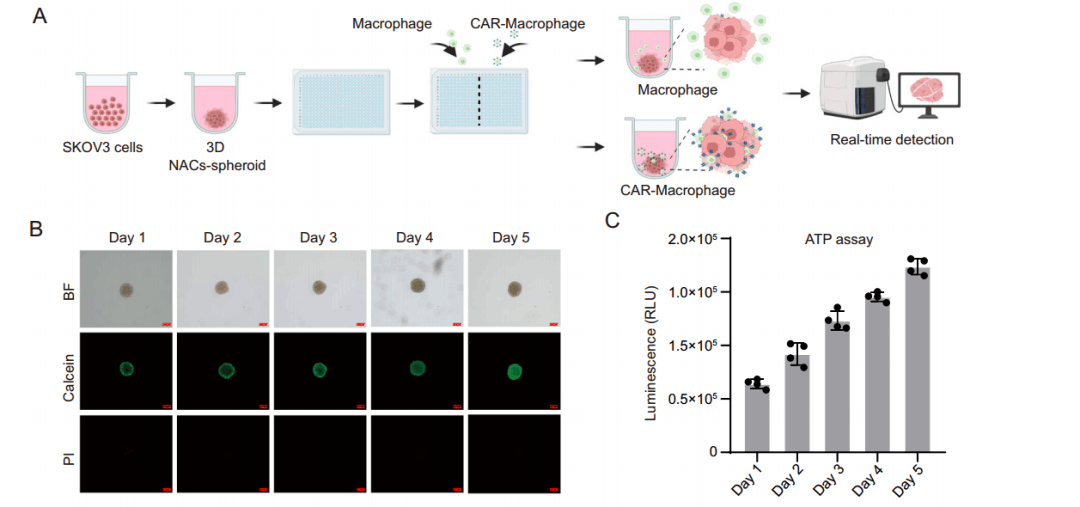
To validate the recognition, infiltration, and cytotoxicity of HER2 CAR-M cells against solid tumors, the team generated a 3D ovarian tumor spheroid model using SKOV3-Fluc cells. They co-cultured UTD-M cells and HER2 CAR-M cells with these spheroids to evaluate infiltration and cytotoxicity across different effector-to-target (E/T) ratios and durations. The findings revealed that HER2 CAR-M cells could effectively infiltrate tumor spheroids at various E/T ratios, with optimal performance at 10:1. Conversely, UTD-M cells displayed only minimal targeting and limited penetration into the spheroids. At the 10:1 E/T ratio, CAR-HER2-M cells demonstrated markedly greater cytotoxicity against solid tumor spheroids compared to UTD-M cells, with this improved infiltration and cytotoxicity becoming more pronounced over time.
By integrating NAC-Organ technology with CAR-M cell therapy, this research systematically assessed CAR-M cells' targeting and cytotoxicity against solid tumors in a 3D ovarian cancer model. It proposes an innovative solution to address the complex challenges of solid tumor treatment. The findings not only establish a robust scientific foundation for the clinical application of CAR-M cells but also offer new insights and approaches for personalized and precision medicine.
HTML Maker